JAMA. 2000;284:1644-1646.
From
Morbidity & Mortality Weekly Report (MMWR)
Human Anthrax Associated With an Epizootic
Among Livestock --- North Dakota, 2000
[MMWR
50(32):677-680, 2001. Centers for Disease Control]
On August 28, 2000, the North Dakota Department of Health was notified by a local clinician of a patient with a cutaneous lesion suggestive of anthrax following exposure to an infected animal carcass. This report summarizes the investigation of this case, which was associated with an anthrax epizootic among livestock in North Dakota, and emphasizes the importance of increased vigilance for human cases of anthrax during and following outbreaks of anthrax among livestock.
On August 19, 2000, a 67-year-old resident of eastern North Dakota participated in the disposal of five cows that had died of anthrax. On the day of disposal, he placed chains around the heads and hooves of the animals and moved them to a burial site. He reported having worn leather gloves throughout transportation and disposal.
On August 23, he noticed a small bump on his left cheek at the angle of his jaw. On August 25, the lesion had enlarged and he sought medical attention. He denied fever, malaise, headache, pruritus, or difficulty swallowing. On examination, the lesion was indurated to approximately the size of a quarter and was surrounded by a purple colored ring. The patient was afebrile and did not appear ill. The physician reported a firm, nontender, superficial nodule with an overlying 0.5 cm black eschar. No drainage was noted and neither wound nor blood cultures was obtained. The patient was placed on ciprofloxacin 500 mg twice a day for presumed cutaneous anthrax.
On follow-up examination on August 28, the eschar had enlarged to 1 cm. Following consultation with the North Dakota Department of Health and based on clinical suspicion of anthrax, the patient continued the course of ciprofloxacin for a total of 14 days. The lesion slowly improved over several weeks. Paired serum specimens were obtained on September 22 and October 5, 2000, and were tested at CDC; both had positive antibody titers by ELISA of 200 to protective antigen, confirming infection with Bacillus anthracis.
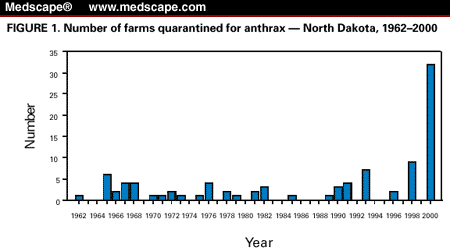
This case was associated with an anthrax epizootic in North Dakota, during which 32 farms were quarantined for anthrax in 2000*, compared with an average of two farms per year during the preceding 40 years (Figure 1). The initial cases were detected in May 2000, when four animals were found dead on a farm; the deaths were later confirmed to be associated with anthrax. During the epizootic, which extended from July 6 through September 24, 2000, 157 animals died on 31 farms on which 62 persons were involved with animal care, vaccination, specimen processing, or carcass disposal. No other cases of symptomatic anthrax were identified in humans in North Dakota.
Reported by: L Shireley, MPH, T Dwelle, MD, D Streitz, North Dakota Dept of Health; L Schuler, DVM, North Dakota Dept of Agriculture. Animal and Plant Health Inspection Svc, US Dept of Agriculture. Meningitis and Special Pathogens Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; and an EIS Officer, CDC.
Editorial Note This report presents the first case of cutaneous anthrax in the United States since 1992. In the United States, the annual incidence of human anthrax declined from approximately 200 cases in the early 1900s to no human cases since 1992. Although most cases reported in the United States have been cutaneous, 18 cases of inhalational anthrax were reported during the 20th century, most recently in 1976 [1]. No cases of gastrointestinal anthrax have been reported in the United States.
Anthrax most commonly occurs in both wild and domestic mammals (e.g., cattle, sheep, goats, camels, antelopes, and other herbivores) [2]. Humans develop anthrax infection following exposure to infected animals, tissue from infected animals, or by direct exposure to B. anthracis [3,4]. Exposure to infected animal tissue can occur during postmortem examination, slaughter, or handling of infected meat or hides. Exposure also can occur during laboratory manipulation of infected blood, muscle, or other tissues. Human-to-human transmission of anthrax is rare.
Anthrax can occur in three forms: cutaneous, gastrointestinal, and inhalational [2]. Most cases (95% worldwide) are cutaneous. The incubation period for cutaneous anthrax ranges from 12 hours to 12 days [2--5]. Cutaneous anthrax may begin with pruritus at the affected site, typically followed by a small, painless papule that progresses to a vesicle in 1--2 days. The lesion erodes, leaving a necrotic ulcer with a characteristic black center. Secondary vesicles are sometimes observed, lymphadenopathy may occur, and local edema may be extensive. Patients may have fever, malaise, and headache. The most common sites of cutaneous anthrax are the hands, forearms, and head. Of the 203 cases reported in the United States since 1955 in which the site of infection was known, 64 (27%) have been in the head and neck region [2]. Presumably, the mechanism of inoculation in this case was the transfer of infective spores on the patient's gloves to broken skin on his face.
Untreated, 20% of persons with cutaneous anthrax die, compared with <1% of those who receive antibiotic therapy [2,6]. B. anthracis is sensitive in vitro to penicillin, tetracycline, chloramphenicol, and ciprofloxacin [7]. In localized or uncomplicated cases of cutaneous anthrax, the recommended regimen is penicillin V, 500 mg taken orally every 6 hours for 5--7 days. For more severe cases of cutaneous anthrax, penicillin G, 4--6 million units every 6 hours intravenously for 7--10 days is recommended. Doxycycline, 100 mg twice a day for localized cases or intravenously for serious cases, also can be used [7--9].
Veterinarians and agricultural workers should minimize direct contact with animals suspected to have died of anthrax. For confirmation by smear or culture, the carcass should not be opened, and a postmortem blood sample should be obtained aseptically by a veterinarian from an accessible peripheral vein (e.g., jugular vein). Specimens also can be obtained from hemorrhagic nasal, buccal, or anal exudate or from materials contaminated with the exudate. If possible, the carcass should be burned or buried where it is found. To minimize environmental contamination, burning is the preferred disposal method. Bedding and other materials found around the carcass (e.g., contaminated soil) also should be burned or buried, and all remaining animals should be promptly removed from the affected pasture. Farms where anthrax deaths among livestock are confirmed should be quarantined and all susceptible healthy livestock on the affected and neighboring premises vaccinated with the Sterne vaccine. Where anthrax is suspected or confirmed, use of long-acting antibiotics followed by vaccination may be effective in reducing livestock deaths. However, this regimen has not been systematically evaluated.
Because this epizootic may continue in North Dakota and because anthrax cases among livestock occur each year, health-care providers should consider the possibility of anthrax when evaluating patients with characteristic skin lesions, particularly if the exposure history includes handling of animals with confirmed or suspected anthrax. Vigilance for human cases of anthrax should be heightened during anthrax epizootics. Veterinary health services should work closely with public and private health officials to ensure early detection and treatment of possible human anthrax cases resulting from exposure to animals during an epizootic. Any person who handles carcasses of animals that have died or are suspected to have died of anthrax should contact their health-care provider if they develop a skin lesion. Although veterinarians, agricultural workers, and laboratory workers might be at increased risk for B. anthracis infection during these epizootics, the risk is low and anthrax vaccination is not recommended[10].
--------------------------------------------------------------------------------
References
1. Brachman PS. Inhalational anthrax. Ann
NY Acad Sci 1980;353:83--93.
2. Brachman PS, Kaufmann A. Anthrax. In: Evans AS, Brachman PS, eds. Bacterial
Infections of Humans. New York, New York: Plenum Medical Book Company,
1998.
3. Bell JH. On anthrax and athracaemia in wool sorters, heifers, and sheep.
Br Med J 1880;2:656--61.
4. Davies JC. A major epidemic of anthrax in Zimbabwe. Cent Afr J Med
1982;28:291--8.
5. Turnbull PCB. Guidelines for the surveillance and control of anthrax
in humans and animals. Geneva, Switzerland: World Health Organization,
1998;(publication no. WHO/EMC/ZDI/98.6).
6. Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med
1999;341:815--26.
7. Lightfoot NF, Scott RJD, Turnbull PCB. Antimicrobial susceptibility
of Bacillus anthracis. Salisbury Med Bull 1990;68:95--8.
8. Barnes JM. Penicillin and B. anthracis. Journal of Pathology and Bacteriology
1947;194:113--25.
9. Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition
and management of patients exposed to biological warfare agents. JAMA
1997;278:399--411.
10. Ashford DA, Rotz LD, Perkins BA. Use of anthrax vaccine in the United
States: recommendations of the Advisory Committee on Immunization Practice
(ACIP). MMWR
2000; 49(no. RR--15).
--------------------------------------------------------------------------------
* A quarantined farm is one on which at least one case of culture-confirmed anthrax has occurred among livestock.
† All MMWR references are available on the Internet Use the search function to find specific articles
J Commun Dis 2000 Dec;32(4):240-6
Human anthrax in India: urgent need for effective prevention.
Kumar A, Kanungo R, Bhattacharya S, Badrinath S, Dutta TK, Swaminathan RP. Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry-605 006, India.
Anthrax is a zoonotic illness caused by Bacillus anthracis. Sporadic cases continue to be reported from many parts of the world. From India, both sporadic cases and outbreaks are being reported regularly. The Union Territory of Pondicherry (a former French colony) lies on the coast of Bay of Bengal, where the incidence of anthrax is on the rise with 28 cases being detected in the year 1999 and 2000 alone. So far, about 34 human cases have been encountered in this region. Recently, an increase in the number of anthrax cases has been noted in veterinary and human practice in this area. Most cases have occurred in agricultural labourers who gave history of handling animal meat or skin of infected animals. The meningitic form of the disease has a very bad prognosis. Patients with this form of disease died despite treatment with high dose penicillin. The typical bacilli were seen in the CSF in all cases of anthrax meningitis and was diagnostic of the condition. The cutaneous form of illness had a benign course and responded favourably to penicillin treatment. Awareness among clinicians and mandatory reporting of cases to public health departments along with public education will help control morbidity and mortality due to anthrax. Effective immunization of animals is the other important control measure for anthrax.
PMID: 11668934
J Appl Microbiol 1999 Aug;87(2):196-9
Anthrax explodes in an Australian summer.
Turner AJ, Galvin JW, Rubira RJ, Miller GT. Department of Natural Resources and Environment, Attwood, Victoria, Australia. Andrew.Turner@nre.vic.gov.au
Anthrax occurred on 83 properties in an area of north central Victoria between 26 January and 26 March in the summer of 1997. Anthrax had not been recorded in the outbreak area since records were initiated in 1914, although anthrax did occur in the general area in the 1880s to 1890s. Standard Australian control measures were applied to the properties, including quarantine, tracing movements of animals on and off affected properties, secure disposal of carcases by burning, enhanced surveillance of stock generally in the area and the use of local disaster control procedures including an alert of health authorities. As affected property numbers began to increase dramatically from 8 February, it was decided to use blanket area vaccination to control the disease. By 26 February, the epidemic curve had returned to the base line and a buffer vaccination zone of 457 farms holding 78,649 cattle was formed by early March 1997. Between 26 January and 26 March when the outbreak was declared over, 202 cattle and 4 sheep were confirmed to have died of anthrax. Between 27 March and early November a further 26 cattle were confirmed as dying due to anthrax and 14 of these had not had previous vaccination, including four young calves and one horse. One new property within the vaccination buffer zone had an anthrax case in a cow in early November 1997. By mid-November 1997, all previously infected and all neighbouring properties within 1 km were compulsorily re-vaccinated, as were all calves when two months of age and all introduced cattle. In 1998, only two confirmed cases of anthrax were diagnosed; both were vaccinated calves on farms which had had multiple cases during the outbreak. The public reaction and attention fueled by unprecedented media attention led to intense international scrutiny from countries where anthrax is a particular zoonotic problem. Very strong representations had to be made about the safety of livestock and livestock products that came from Victoria. This event has demonstrated that there is a need to review OIE and other requirements and recommendations covering anthrax where strict restrictions are placed on livestock and livestock products to protect livestock and human populations against anthrax infection.
PMID: 10475947
J Appl Microbiol 1999 Aug;87(2):189-91
1996-97 Global Anthrax Report.
Hugh-Jones M. Department of Epidemiology & Community Health, School of Veterinary Medicine, Louisiana State University, Baton Rouge 70803, USA.
While there is a general decrease in the number of anthrax outbreaks, and thus of human cases, worldwide this is still a disease that is extensively under-diagnosed and under-reported. However, it is now very infrequent to rare in Canada, the United States, and many countries in Europe. An increasing number of countries are now free. At the other extreme, it is a significant problem in West Africa, Spain, Greece, Turkey, Albania, Romania and in Central Asia. In spite of the textbooks, livestock and wildlife deaths do occur, sometimes commonly, without any 'diagnostic' extravasation of blood and, if not realised, infected carcasses get recycled into meat and bone meals for feed.
PMID: 10475945
Semin Respir Infect 1997 Mar;12(1):28-30
Anthrax pneumonia.
Penn CC, Klotz SA. University of Kansas School of Medicine, Kansas City, USA.
Inhalation anthrax is a rare and almost uniformly fatal form of human anthrax caused by the inhalation of spores of Bacillus anthracis. A clue to the diagnosis is provided by taking a work history which will disclose patient exposure to contaminated animal products, most often animal hair and wool used in the textile industry. It is an illness with a biphasic course marked by the presence of a widened mediastinum on chest radiograph and often accompanied by hemorrhagic meningitis. The pathogenesis of this disease as well as the differential diagnosis of inhalation anthrax in the context of other zoonotic pneumonias is discussed. Therapy has been ineffectual probably because it has begun too late, but includes intravenous high dose penicillin G and perhaps vaccination to prevent relapse.
PMID: 9097373
South Med J 1993 Jan;86(1):1-4
Indigenous human cutaneous anthrax in Texas.
Taylor JP, Dimmitt DC, Ezzell JW, Whitford H. Epidemiology Division, Texas Department of Health, Austin 78756.
In December 1988 an indigenous case of cutaneous anthrax was identified in Texas. The patient, a 63-year-old male Hispanic from southwest Texas, was a sheep shearer and had a recent history of dissecting sheep that had died suddenly. He experienced an illness characterized by left arm pain and edema. A necrotic lesion developed on his left forearm, with cellulitis and lymphadenopathy. After treatment with oral and intravenous penicillins, the patient fully recovered. Western blot testing revealed a fourfold or greater rise in antibody titer to Bacillus anthracis protective antigen and lethal factor. This represents the first case of indigenous anthrax in Texas in more than 20 years.
PMID: 8420007
J Am Vet Med Assoc 1977 Feb 1;130(3):327-33
An epizootiologic study of anthrax in Falls County, Texas.
Fox MD, Boyce JM, Kaufmann AF, Young JB, Whitford HW.
In June and July, 1974, an anthrax epizootic in Falls County, Texas, resulted in the death of 236 animals (228 cattle, 5 horses, 2 mules, and 1 pig) on 48 premises. Death rates were highest for horses (18.2%) and bulls (16.8%). The epizootic was apparently precipitated by drought, and infection appeared to be the result of ingesting intrinsically contaminated soil and grass. Human illness was not associated with the epizootic.
PMID: 401803
Am Vet Med Assoc 1975 Nov 1;167(9):842-3
Epizootic of anthrax in Falls County, Texas.
Young JB.
An epizootic of anthrax in Eastern Falls County, Texas, killed at least 238 animals during a 6-week period ending July 31, 1974. Infection appeared to be caused by the ingestion of contaminated soil and grass in the drought-stricken central Texas area. The participation of the Texas Department of Agriculture, the Texas National Guard, and other state agencies was of great assistance to the Texas Animal Health Commission in handling the epizootic and panic stricken public. Use of the unencapsulated Sterne strain spore vaccine was credited with preventing many livestock losses in the area. Contaminated city water was detected during the epizootic but human disease did not result.
PMID: 1184447